

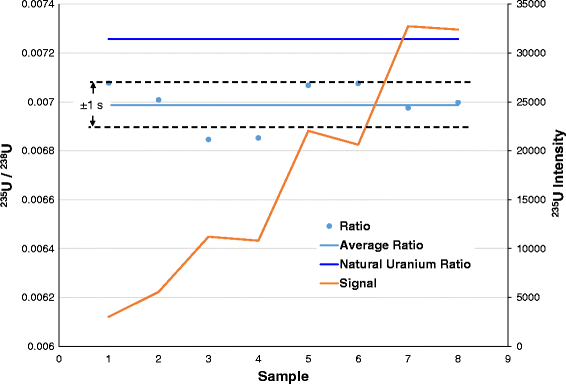
In a nuclear reactor the uranium fuel is assembled in such a way that a controlled fission chain reaction can be achieved. In a nuclear power station, however, the fissioning of uranium atoms replaces the burning of coal or gas. Both require heat to produce steam to drive turbines and generators. Nuclear power stations and fossil-fuelled power stations of similar capacity have many features in common. The heat is used to make steam to produce electricity. It is this process, in effect 'burning' uranium, which occurs in a nuclear reactor. When this happens over and over again, many millions of times, a very large amount of heat is produced from a relatively small amount of uranium. If enough of these expelled neutrons cause the nuclei of other U-235 atoms to split, releasing further neutrons, a fission 'chain reaction' can be achieved. When the nucleus of a U-235 atom captures a moving neutron it splits in two (fissions) and releases some energy in the form of heat, also two or three additional neutrons are thrown off. The nucleus of the U-235 atom comprises 92 protons and 143 neutrons (92 + 143 = 235). Nevertheless it generates 0.1 watts/tonne as decay heat and this is enough to warm the Earth's core. This means that it is barely radioactive, less so than many other isotopes in rocks and sand. U-238 decays very slowly, its half-life being about the same as the age of the Earth (4500 million years). Meanwhile, like all radioactive isotopes, they decay. It is therefore said to be 'fissile' and we use the expression 'nuclear fission'.

The isotope U-235 is important because under certain conditions it can readily be split, yielding a lot of energy.
Natural uranium as found in the Earth's crust is a mixture largely of two isotopes: uranium-238 (U-238), accounting for 99.3% and uranium-235 (U-235) about 0.7%. These isotopes differ from each other in the number of uncharged particles (neutrons) in the nucleus. Like other elements, uranium occurs in several slightly differing forms known as 'isotopes'. On a scale arranged according to the increasing mass of their nuclei, uranium is one of the heaviest of all the naturally-occurring elements (hydrogen is the lightest).


 0 kommentar(er)
0 kommentar(er)
